“This doesn’t smell bad so I don’t need a respirator.” I hear that from time to time, either from students or in online forums. Prop makers working with chemicals use their sense of smell to determine how dangerous something is. “This smells better than that, so I don’t use that anymore.” “I can’t smell a thing, so this must be safe.”
No no no. This is dangerous, and the wrong way to think about safety with chemicals.
For every chemical, OSHA sets limits as to how much you can be exposed to. They try to figure out the amount you can be exposed to while working with something your entire life, and never have adverse health effects from it. These are called Threshold Limit Values (TLV).
The first is  the time-weighted average (TWA). The TWA is meant to indicate what you are constantly exposed to at work. They measure the average amount you are exposed to over an 8-hour day and a 40 hour week. (Uh oh, we often work much more than that in theatre).
Next is the short-term exposure. or STEL. They define this as 15 minutes of exposure. And you have to have an hour break before the next exposure. And you can only have four exposures per day.
Finally there is the ceiling value. You should never reach this level of exposure, even for an instant. They also have IDLH, which is “immediately dangerous to life and health”. Instant exposure at this amount will kill or irreversibly affect your health.
So let’s look at the following chart, which has the TLVs for some common chemicals found in the props shop. You probably recognize some of these as ingredients in paints and coatings. Amines are found in many epoxies. Methyl ethyl ketone is used in polyester resin. The diisocyanates (TDI and MDI) are two of the more common curing agents used in two-part polyurethanes.
All the values are measured in parts per million (ppm), which means out of a million pieces of air, that is how many pieces are of the substance being measured (for comparison, room air has 209,500 ppm of oxygen).
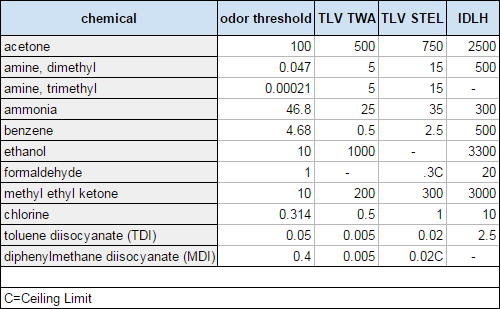
So where does smell come in? Well, every chemical has an “odor threshold”. This is the amount, again in ppm, of a chemical at which point you can smell it. This is much less standardized, because it can be hard to test and different people have different sensitivities to smell. The number is often given in a range. You can see in the chart that the odor thresholds are all over the place for the different chemicals.
I’ve pulled a few chemicals out and put them in a chart so you can see what’s happening a bit easier.
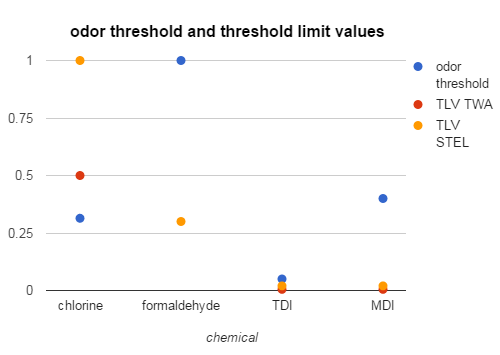
Look at chlorine. The odor threshold is way below the TLV TWA. This means that even if you smell chlorine, you may not be exposed to a harmful amount. You may be able to smell chlorine all day every day and still not have harmful effects (like if you work at an indoor pool).
Now look at formaldehyde. The short-term exposure limit (the orange dot) is way down in the graph. The odor threshold is way at the top. That means you can be exposed to a harmful amount before smelling it. In fact, you will have to be exposed to three times the threshold limit before you can smell it. So if you are working with something that off-gasses formaldehyde (including many plywoods and engineered lumber, VOC-containing paints, and even some fabrics), you cannot assume you are safe because you do not smell anything.
Look at the two diisocyanates (MDI and TDI). Both of them also have an odor threshold above their short-term exposure limit. If you look back at the chart, you will see that the STEL for MDI is also its ceiling value, which is the amount you should not exceed even for an instant. And its odor threshold is twenty times higher than that. You can be breathing dangerous and even deadly amounts of MDI before you even get close to smelling it.
This is why many people suggest casting urethane parts inside a fume hood or a spray booth; even a respirator is not a reliable protector. One way to tell if your respirator has stopped working is if you can smell the outside air. But with these chemicals, you cannot smell them even when they are present in dangerous amounts. So you have no indication of whether your respirator is working or not.
Your nose is a great sensor for many chemicals, but you should never rely solely on it for your safety. You need to know about the specific chemicals you are working with and how their odor threshold relates to their threshold limit values. No more, “this doesn’t smell bad so I don’t need a respirator.”
Smell you later.